At the crossroads of metabolism, immunity, and aging
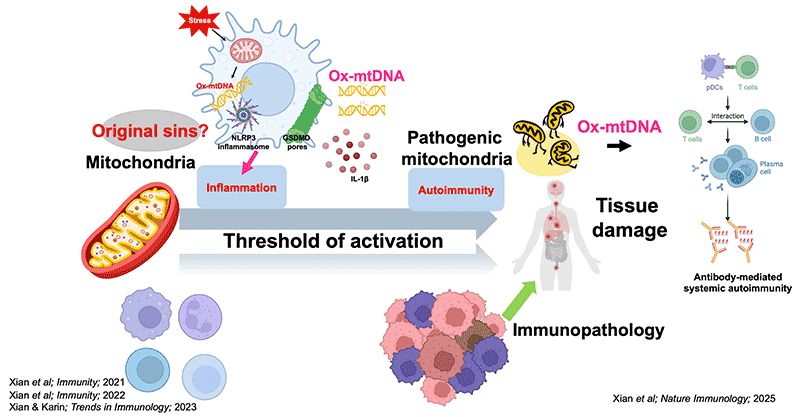
The Xian lab’s long-term goal is to enhance immune therapies by uncovering how mitochondrial stress activates the immune system. We focus on how mitochondria-released danger signals—known as danger-associated molecular patterns (DAMPs)—shape immunity. In particular, we study circulating oxidized mitochondrial DNA (Ox-mtDNA), that rises with aging and correlates with autoimmune, metabolic, and neurodegenerative disorders. By defining these pathways, we aim to translate basic discoveries into new diagnostics and therapies for autoimmunity, aging-related conditions, and cancer.
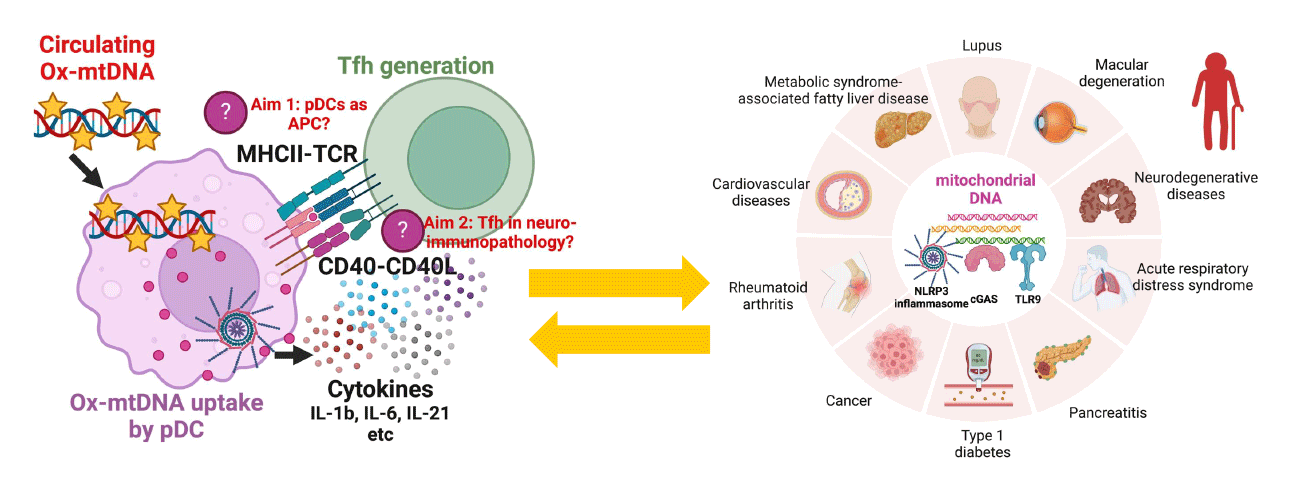
Our recent work revealed that Ox-mtDNA controls the transition from inflammation to autoimmunity, through direct communication between plasmacytoid dendritic cells (pDCs) and T follicular helper (Tfh) cells (Fig. 1). This finding opens two central questions for our lab (Fig. 2):
- Do pDCs present self-antigens to activate Tfh cells in autoimmune disease?
- Are Tfh cells pathogenic in aging and neurodegenerative conditions where circulating Ox-mtDNA is elevated?
To address these questions, we combine human and mouse models with approaches in immunology, molecular genetics, biochemistry, cell biology, and bioinformatics. Through this integrated strategy, we will define the molecular mechanisms of immune pathogenesis and ultimately develop new diagnostic and therapeutic strategies for autoimmunity, neurodegeneration and cancer.